In the summer of 2018, the country was shaken by an unusual incident – a two-month-old boy from the Gantsevichi region was vaccinated with Eupent vaccine, which was produced by a South Korean pharmaceutical company. The kid began to choke and soon died. Investigation into his death was taken up by the editor-in-chief of the newspaper “Ezhednevnik” Sergei Satsuk. In 2020, a criminal case was launched against him for this, he had to spend some time in prison. Let’s find out what has been revealed about the Eupenta vaccine and how it relates to the prosecution of Sergei Satsuk.
Details of the tragedy in the Gantsevichi region
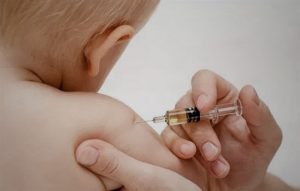 Two-month-old Kirill lived with his mother and father in the village of Bolshiye Krugovichi. On August 13, 2018, an employee of the Ogarevich outpatient clinic came to the family’s home to give the baby a routine vaccination. The boy received two vaccines: Korean Eupenta and French Immovax Polio. The medical worker was experienced, with over 30 years of work experience, said the Gantsevichi Central District Hospital. While still in the obstetrics ward, Kirill’s mother noticed that the baby had gone pale and was panting. The child was given adrenaline and an ambulance was called.
Two-month-old Kirill lived with his mother and father in the village of Bolshiye Krugovichi. On August 13, 2018, an employee of the Ogarevich outpatient clinic came to the family’s home to give the baby a routine vaccination. The boy received two vaccines: Korean Eupenta and French Immovax Polio. The medical worker was experienced, with over 30 years of work experience, said the Gantsevichi Central District Hospital. While still in the obstetrics ward, Kirill’s mother noticed that the baby had gone pale and was panting. The child was given adrenaline and an ambulance was called.
The distance from the Gantsevichi Central District Hospital to the Medical and obstetric unit, where Kirill was vaccinated, is 13.6 kilometres. On the way to the hospital in the ambulance, the boy was given an injection – it is not known which one. After that, saliva and blood flowed from the child’s mouth. The team urgently delivered the baby to the hospital, and the doctors began to rescue him. But they failed to bring Kirill back to life.
Alexander, the father of the deceased baby, told “Komsomolskaya Pravda” that his son was born absolutely healthy. The boy’s mother was checked during pregnancy, did an ultrasound. Before the vaccination, the child was also examined by a doctor and did not reveal any abnormalities.
After Kirill’s death, doctors performed an autopsy. It turned out that the baby was absolutely healthy, no diseases or abnormalities were revealed, and the cause of death was multiple organ failure, which was caused by severe anaphylactic shock.
On the same day, another little girl from Gantsevichi, Ulyana, received the exact same vaccine. After the vaccination, the baby had a fever, and her mother called doctors. The girl was taken to the hospital, and a few days later, without revealing any abnormalities, she was discharged.
What is Eupenta?
 Eupenta is a five-component vaccine for the prevention of diphtheria, tetanus, pertussis, hepatitis B, and haemophilus infections. Thus, this vaccine will be able to protect children from five infections at once. It was produced in South Korea at LG Chem. The company has a certificate of conformity of production with the requirements of Good Manufacturing Practice.
Eupenta is a five-component vaccine for the prevention of diphtheria, tetanus, pertussis, hepatitis B, and haemophilus infections. Thus, this vaccine will be able to protect children from five infections at once. It was produced in South Korea at LG Chem. The company has a certificate of conformity of production with the requirements of Good Manufacturing Practice.
The Eupenta vaccine is also recommended and purchased by the UN International Children’s Fund UNICEF. In Belarus, Eupenta has been actively used on our children since January 2017. At the same time, the vaccine was not registered in Belarus and, accordingly, did not pass any tests and checks in our country at all. The use of such a vaccine is strictly prohibited by the “Medicines Act”, but it has been actively used for more than a year and a half.
Why did they start using an unregistered vaccine in Belarus?
The Ministry of Health claims that the “Medicines Act” provides a procedure for the importation of unregistered medicines.
“If the purchase of an unregistered drug is required, then additional requirements are imposed on such drugs. The medicine must be registered in countries that are members of the ICH (International Conference on Harmonisation of Technical Requirements for Registration of Medicinal Products for Human Use). This list includes countries with a strong regulatory body for registration (EU countries, USA, Canada, Japan, Switzerland, South Korea, etc.),” the Ministry of Health said.
However, in his investigation, Sergei Satsuk clarified that the massive import of the vaccine could occur only in two cases: for conducting clinical (preclinical) trials and for eliminating the consequences of natural and man-made emergencies, epidemic diseases. The rest of the cases are private and cannot be attributed to this vaccine. However, there were no epidemics in Belarus, therefore, the vaccine could be imported for clinical (preclinical) trials.
And here the most eerie begins. Participation in clinical trials is purely voluntary. A lot of money is paid for this, and compensation is also provided if something suddenly goes wrong. Pharmaceutical companies spend millions or even tens of millions of dollars on clinical trials. And here a Korean company was given a free trial run of Belarusian children. Or maybe not completely free?
How the vaccine is registered?
The development, testing and marketing of a new drug is extremely expensive. World pharmaceutical companies spend 10-15 years and up to $ 800 million on this. At the same time, the wider the scope of the drug, the more difficult its tests and the more funds are required for them. The new drug is first tested on animals (necessarily on two different species) and only after that clinical trials on humans begin, which include three phases:
– The first phase of testing is carried out on a small number of people, moreover, always on healthy adult volunteers.
– The second phase is testing on the target group of people, that is, on those for whom the drug is intended. There are already up to 300 volunteers participating, who are under close medical supervision.
– The third phase is massive clinical trials, which, in turn, can include several stages, where thousands of people are involved.
Only after the third phase of clinical trials begins the process of drug registration, confirming its effectiveness and safety. Moreover, if some unfavorable data were obtained during clinical trials, the drug is unlikely to be registered.
The delegation of the Korean company presented the Ministry of Health of Belarus with a pharmaceutical product certificate issued by the Korean national regulator Ministry of Food and Drug Safety. As noted by the Ministry of Health, this certificate number 5115 dated May 27, 2014 confirms that the Eupent vaccine has passed all the necessary tests, therefore it is safe and effective. However, on the PHARMA KOREA website, where there is all the information about medicines registered in the country and in development, it is stated: Eupenta is not even registered in the manufacturing country. The vaccine is still in the scientific development section, just at the registration stage. Moreover, in 2014, it was produced at the Iksan Plant, and in 2016, production was moved to the new Osong Campus site, which automatically cancels all previously issued registrations, since the production site was not inspected. What certificate the Korean company provided to the Ministry of Health is unknown.
Commentary of the Ministry of Health – where is the truth, and where is the lie
 It turned out that Eupenta’s tests were carried out in 2015 and 2017 in several countries around the world. However, the drug has not been registered in any of the countries. But in all these countries, the tests were carried out exclusively on volunteers who were paid a lot of money and who were under constant medical supervision. But there is no information anywhere about clinical trials in Belarus, and the Ministry of Health speaks of 50 million successful cases of vaccine use. But at the same time, it is not clear why the registration certificate was never issued to her. Moreover, the Internet on various forums and sites is full of mothers’ comments about the child’s very negative reaction to Eupenta.
It turned out that Eupenta’s tests were carried out in 2015 and 2017 in several countries around the world. However, the drug has not been registered in any of the countries. But in all these countries, the tests were carried out exclusively on volunteers who were paid a lot of money and who were under constant medical supervision. But there is no information anywhere about clinical trials in Belarus, and the Ministry of Health speaks of 50 million successful cases of vaccine use. But at the same time, it is not clear why the registration certificate was never issued to her. Moreover, the Internet on various forums and sites is full of mothers’ comments about the child’s very negative reaction to Eupenta.
In its commentary, the Ministry of Health said that in order to import an unregistered drug into a country, a drug must be registered in the countries of the ICH. However, ICH does not include any country in the world. Within the framework of this organization, there are no intercountry agreements and obligations, just as there is no and cannot be, in principle, mutual recognition of registration of medicines. The purpose of the organization is not the recognition of mutual registration, but only the development of uniform approaches to the quality control of drugs. And in the “Medicines Act” there is not a word about ICH, as well as about WHO prequalification and the fact that it allows you to import medicines without registration in Belarus. After all, the WHO prequalification program was created with the aim of simplifying the admission of medicines to developing countries that do not have their own structures involved in the quality control of medicines.
According to officials of the Ministry of Health, the decision to purchase Eupenta was made after the manufacturer of the DPT vaccine from the Russian Federation, which for a long time provided Belarus with immunobiological preparations, was unable to ensure the supply of the vaccine of adequate quality on time. They say that is why, “in order to prevent an emergency,” in particular, the manifestation of dangerous infectious diseases, of which 80% are fatal, and the decision was made to purchase the Eupent vaccine.
It turned out that in Belarus there really was a difficult situation with the supply of vaccines and the implementation of the national immunization schedule – mothers of many babies complained about this. The Ministry of Health explained: the Russian company “Microgen” was unable to supply the DPT vaccine of proper quality to Belarus. In “Microgen” they explained: there were no complaints about the quality of DPT, and the registration of the vaccine in Belarus is valid until May 2020.
Information about the purchase of the Eupent vaccine by the UN International Children’s Fund UNICEF is available on the website of the Ministry of Health of Belarus. On the website of The Korea Herald, there is information that the vaccine manufacturer in 2016 expressed hope of supplying the vaccine to UN organizations, in particular UNICEF. On October 20, 2016, the Business Korea website reported that LG Chem had entered into a deal for the supply of Eupents for UNICEF from 2017 to 2019. This vaccine should have been given to about 30 million babies. The manufacturers clarified that the vaccine passed the relevant World Health Organization prequalification standards in February 2016.
How did Eupenta get to Belarus?

Dmitry Pinevich
Sergei Satsuk analyzed vaccine purchases: in December 2016, an auction for the purchase of DPT was held, which was carried out on behalf of the First Deputy Minister of Health Dmitry Pinevich. Among others, the DPT vaccine was on the list. But what is surprising is that the purchase volume of the vaccine, which is stored for only 18 months, was indicated in more than half a million doses! At the same time, the birth rate in Belarus in 2017 amounted to only 111 thousand children – by the way, it can be calculated quite accurately in advance. By announcing the auction for the purchase of the vaccine, “Belpharmacia” set exactly the volumes that the Ministry of Health ordered.
Thus, Microgen really would not be able to supply the entire volume of the vaccine. After two failed auctions, the process turned into a single-source procurement procedure, under which the unregistered Eupenta was acquired. And of course, the volume was not half a million doses, but 2.5 times less – 200 thousand doses. At the same time, other vaccines, for example, the French “Pentaxim” of good quality, were ignored.
Initially, the vaccine was procured on a competitive basis. The conditions stated that if an unregistered medicinal product is applied for the competition, then the participant must provide a letter of guarantee “on the implementation of the state registration of drugs on the territory of the Republic of Belarus (with the attachment of an agreement with the REPUBLICAN UNITARY ENTERPRISE “CENTRE FOR EXPERTISE AND TESTING IN HEALTH CARE”), that is, after the victory, he had to register in Belarus. But there was no registration of Eupenta.
Previously, registration was denied to drugs that lacked one comma in the document, which the manufacturers complained about. Even if a medicine is registered and has been supplied to Belarus for many years, an incorrectly executed instruction will become a reason for withdrawing the medicine from circulation. Eupenta’s instructions, on the other hand, have many flaws: instead of indicating a specific expiry date it says “not expired”, the column “Formulation” shows the manufacturer, and the column “Manufacturer” shows sources of raw materials and date of issue is unclear, the column “Side effects” does not describe the side effects of this particular medicine. It is nonsense for Belarus that a drug with such an instruction, without a description of side effects, was released on the market. Such a drug, according to Belarusian legislation, is a priori considered to be of poor quality and is subject to a ban on distribution.
Ministry of Health’s response to the allegations
Representatives of the Ministry of Health tried to explain the situation with the purchase and use of the unregistered Eupent vaccine, but it came out unconvincing. According to them, a problem with the quality of vaccines was revealed almost every year. Certain series of DPT vaccines were not allowed for medical use in our country. In November, in order to ensure a reliable guaranteed supply, the Ministry of Health approached the leading manufacturers of registered vaccines, which include components of whooping cough, diphtheria, and tetanus, with a request to find the possibility of prompt delivery of the necessary vaccines. The Ministry of Health was simultaneously working on the possibility of separate deliveries of DPT vaccine from other countries. The countries were China, India and Korea. In November 2017, a decision was made on the public procurement from one source of the pentavalent Eupent vaccine.
Deputy Director of the Centre for Expertise and Testing in Healthcare Sergey Marchenko added that the import and use of the unregistered Eupent vaccine was carried out in accordance with the “Medicines Act”, in particular, Art.23. The questions remain: Why wasn’t the registration of the DPT vaccine cancelled? Why couldn’t the leading vaccine manufacturers bid for the vaccine? There is an answer to this question. More than 574,000 doses were declared for purchase, which of course was difficult to deliver on short notice. The cost for one dose is 40 kopecks. So, the Ministry of Health made a decision to purchase an unregistered Eupent vaccine from one source. The ministry stressed that the Eupent vaccine was imported and approved for use “in order to prevent an emergency situation of outbreaks of dangerous infectious diseases.” At the same time, it is possible to import an unregistered drug according to the law in order to eliminate the consequences of an emergency.
In order for the supplier to be able to import a truck with medicines into the territory of Belarus, he had to provide customs with official registration in the Republic of Belarus, or another document from the Ministry of Health, on the basis of which the delivery is carried out. And here another question arises: what was indicated in this document? It is unlikely that the Ministry of Health indicated in the document “in order to prevent an emergency situation of outbreaks of dangerous infectious diseases.” Customs officers are well aware of the law, they are constantly dealing with deliveries of medicines and it is hard to believe that they “bought” such irrelevant wording.
Korean company statement: true and false
Soon, the Ministry of Health received a vaccine from the manufacturer. It turned out that the official website of the Ministry of Food and Drug Safety (https://ezdrug.mfds.go.kr, also known as KFDA) has correct and up-to-date information that the Eupent vaccine is registered with Korea. The company also sent a copy of the Eupenta pharmaceutical product certificate in 2015 (old version) and 2018 (updated version). What certificate from 2014 the Koreans showed during their arrival is not clear. The Ministry of Health refused to explain this, referring to the fact that “the prescription (full composition) of the finished product is always indicated in the certificate or in the appendix to it – this information reveals in detail the quantitative composition of the ingredients in the product. This information is confidential”.
Serhеy Satsuk notes that certificates confirming the quality and safety of a product are public documents, where there is not and cannot be any secret information. And any control and analytical laboratory will easily find out the quantitative composition of any drug. The journalist also managed to find the official website of the Ministry of Food and Drug Safety – it is located at a different address. According to him, in 2016, the Eupent vaccine was not registered in Korea, and there is no information about the manufacturer at all.
On the website indicated by the Koreans, there is information available only to residents of South Korea. One open form query for the word “Eupenta” did not turn up anything, but it did turn up the certificate from 2014 that the company was talking about. In the “registration / license” column in the vaccine registration information, there was the word “license”, and for export. Consequently, the regulator proceeded from the position that registration will be obtained in the country where the drug will be exported.
The Koreans have attached to the letter a list of countries where Eupenta is registered. These are South Korea, Uzbekistan, Pakistan, Sri Lanka, the Republic of the Union of Myanmar, Syria, Ethiopia, the Philippines, the Democratic Republic of the Congo. None of them, except for South Korea, are included in the list with high regulatory requirements, but almost all of them are a classic WHO contingent, where drugs are supplied under prequalification and UN programs. Registration of medicines in these countries goes like this: if there is an appropriate regulatory body, then it registers a medicine on the basis of WHO prequalification, without documents, only based on trust in the organization.
By the way, on the websites of the ministries of health of Uzbekistan and the Philippines, there was no mention of Eupente.
IC joined the case
 As soon as it became known that the certificate No. 5115 could not be a confirmation of the safety of the Eupent vaccine, the Investigative Committee and the authorities got involved in the case. Another release was immediately published, in which the first certificate is not mentioned in a word (perhaps so that the IC does not withdraw it for verification), but two new certificates appeared. At the same time, officials of the Ministry of Health did not even ask the Koreans why they suddenly changed the certificate and how they can explain the provision of the first.
As soon as it became known that the certificate No. 5115 could not be a confirmation of the safety of the Eupent vaccine, the Investigative Committee and the authorities got involved in the case. Another release was immediately published, in which the first certificate is not mentioned in a word (perhaps so that the IC does not withdraw it for verification), but two new certificates appeared. At the same time, officials of the Ministry of Health did not even ask the Koreans why they suddenly changed the certificate and how they can explain the provision of the first.
In its official statement, which was published by the Ministry of Health, a representative of LG Chem also indicated that the Eupent vaccine was originally produced at the Osong production site, and WHO prequalification is carried out only after three phases of clinical trials and nothing else. The WHO website says that there is only one phase – the results of clinical trials conducted on healthy volunteers.
In 2019, the Investigative Committee announced the results of an audit conducted on the death of a child in the Gantsevichi region, and stated that Eupent’s vaccine was of high quality. And added that “during the inspection, violations of the procurement procedure were established, related to imperfect legislation and dictated by the objective need to purchase a large amount of vaccine as soon as possible.”
Investigators, according to the IC, have studied the documentation confirming the quality of the Eupenta vaccine: including certificates of factory analysis of a drug from South Korea and a certificate issued for the importing country. The IC did not explain how a large number of vaccines needed to be procured on a one-off basis, or why a critical situation with vaccination was artificially created in order to introduce the unregistered Eupenta to the market. The IC did not respond to the lack of confirmation of the effectiveness and safety of the vaccine and did not provide a legal assessment of the actions of the Ministry of Health officials who allowed the vaccine into Belarus without confirmation of effectiveness and safety. But what is most surprising is that the IC said nothing at all about the prequalification of the Eupent vaccine by the World Health Organization.
“Currently, immunization with the Eupenta vaccine continues in Belarus. Moreover, from January to August 2018, about 100 thousand Eupente vaccinations were made, and only in two cases adverse reactions were recorded. The number of such reactions after the introduction of a previously used other vaccine was, on average, several times greater,” the Investigative Committee summed up its inspection. The position of the IC regarding the fact that there is no connection between violations in the procurement procedure and the death of the child is quite surprising, because the side effects of the drug were not considered.
Registration of Eupenta in Belarus
In May 2019, information appeared that the state register of medicines of the Center for Expertise and Testing in Healthcare indicated that the vaccine was registered on March 1. Registration deadline is March 1, 2024. Neither the Ministry of Health, nor the Investigative Committee has ever voiced the grounds on which the vaccine was imported and authorised in Belarus. Moreover, according to the law, it could only be imported for clinical trials.
Who made the experiment on Belarusian children?

Valery Malashko
The Minister of Health in the year when the incident occurred in the Gantsevichi region was Valery Malashko. He took office in January 2017 – at the same time the first vaccinations with the Eupent vaccine began in Belarus. And before that he worked as deputy chairman of the Mogilev regional executive committee. His appearance as head of the Ministry of Health was very strange: in December 2016, Malashko was appointed to the post of Minister of Labor and Social Protection. A month and a half later, Alexander Lukashenko changed his mind and sent him to head the Ministry of Health.
Valery Malashko stayed in office for a little over two years and was remembered for several odious decisions. The first is a prescription contact lens sales project. The innovation was actively discussed in the Ministry of Health in the summer of 2017. Officials proposed to discuss the possibility of selling lenses and glasses by prescription and prohibit the sale of lenses in special machines. Representatives of the Ministry of Health spoke with stories about the dangers of lenses. However, this project was never implemented – the Ministry of Health refused to deal with it.
Also, with Valery Malashko, the Ministry of Health began a project on the digitization of medical records and electronic prescriptions. It was assumed that all patients will have medical records with information about their health throughout their lives, and all medical institutions will have access to such medical records. As a result, doctors had to do double work: fill out both paper and electronic documentation, write out prescriptions by hand, and then enter them into a computer.
Shortly before leaving his post, Malashko announced that instead of therapists in medical centres, there would be general practitioners. The Ministry of Health assumed that this would reduce the load on specialized specialists. During his speech at the World Health Assembly, Malashko promised to reform the work of medical centres, which will set a course for a comprehensive, integrated and patient-centered delivery of health services. However, this approach did not solve the problem with queues to narrow specialists in medical centres.
 Finally, under Malashko, the loudest corruption scandal in the medical field in the history of Belarus happened. On June 25, 2018, the chairman of the KGB, Valery Vakulchik, made a statement that the committee had “uncovered a large-scale corruption scheme in the healthcare sector.” In January 2019, 93 people were involved in the “medical case”: 30 officials of the healthcare system and 63 persons – owners (founders), managers and employees of commercial structures. All were suspected of taking bribes of up to tens of thousands of dollars for matters within their competence. Among the detainees were Deputy Minister of Health Igor Lositsky, director of the Republican Scientific and Practical Centre for Traumatology and Orthopaedics Alexander Beletsky, head physician of the 1st hospital in Minsk Oleg Fomin, head of the Department of Pharmaceutical Inspection and Organization of Drug Supply of the Ministry of Health Lyudmila Reutskaya, as well as several directors of medical companies and clinics. The accused received various punishments – from a fine to seven years in prison. Valery Malashko, commenting on the results of the “case of doctors”, said: the corruption scandal is also the fault of the leadership of the Ministry of Health, but “there is corruption in every society and at every level”. Valeriy Malashko also promised to reform the procurement system and the recruitment system. In September 2018, at a meeting, Lukashenko asked him a question: “Tell me honestly, between you and me: are you capable of running a ministry? If you are not able, it will get worse”.
Finally, under Malashko, the loudest corruption scandal in the medical field in the history of Belarus happened. On June 25, 2018, the chairman of the KGB, Valery Vakulchik, made a statement that the committee had “uncovered a large-scale corruption scheme in the healthcare sector.” In January 2019, 93 people were involved in the “medical case”: 30 officials of the healthcare system and 63 persons – owners (founders), managers and employees of commercial structures. All were suspected of taking bribes of up to tens of thousands of dollars for matters within their competence. Among the detainees were Deputy Minister of Health Igor Lositsky, director of the Republican Scientific and Practical Centre for Traumatology and Orthopaedics Alexander Beletsky, head physician of the 1st hospital in Minsk Oleg Fomin, head of the Department of Pharmaceutical Inspection and Organization of Drug Supply of the Ministry of Health Lyudmila Reutskaya, as well as several directors of medical companies and clinics. The accused received various punishments – from a fine to seven years in prison. Valery Malashko, commenting on the results of the “case of doctors”, said: the corruption scandal is also the fault of the leadership of the Ministry of Health, but “there is corruption in every society and at every level”. Valeriy Malashko also promised to reform the procurement system and the recruitment system. In September 2018, at a meeting, Lukashenko asked him a question: “Tell me honestly, between you and me: are you capable of running a ministry? If you are not able, it will get worse”.
The pursuit of Sergei Satsuk

Sergei Satsuk
In August 2019 (a year after the publication of the vaccine), the state TV channel Belarus-1 broadcast a story accusing Satsuk of writing “custom-made articles”. A certain businessman Viktor Medvedsky claimed that he gave the journalist $ 3,000 for preparing another article on corruption in the Ministry of Health.
In March 2020, Sergei Satsuk wrote a column with the subtitle “Who is spreading panic around the coronavirus, the president or websites and channels?” He expressed doubts about the official number of patients and criticized the inaction of the authorities with regard to supporting business in difficult times.
On 25 March 2020, officers of the Financial Investigation Department of the State Control Committee detained Sergei Satsuk. The reason for the detention was allegedly the receipt of money by Sergei Satsuk to conduct journalistic investigations more than a year ago.
Satsuk has previously stated that he is being threatened due to his anti-corruption investigations. Journalists of “Ezhednevnik” called the arrest of Satsuka “a fragment of the corruptionists’ battle against a man who dared to encroach on their constructed system”. The Belarusian Association of Journalists calls on the authorities to end the criminal prosecution of Satsuka.
On 4 April 2020, the preventive measure against Sergei Satsuk was cancelled. The corresponding decision was made by the Prosecutor General of Belarus. Sergei Satsuk was never charged. People who donated their hard-earned funds to support the journalistic investigations of Sergei Satsuk began to be summoned to the Investigative Committee for interrogation.







